Pasteurization is the treatment of a food or beverage product to make it safe for consumption and to improve its shelf life. Unlike sterilization, which uses high-temperature treatment to eliminate all microorganisms, resulting in a product that can be stored indefinitely at room temperature, pasteurization is carried out at lower temperatures and aims to reduce the overall microbial population to acceptable levels that can be maintained at refrigerated temperatures.
The main purpose of pasteurization is to reduced the “bioburden” of the product. The bioburden is defined as the number of contaminating organisms found in a given amount of material before undergoing a sterilizing or pasteurizing procedure. These organisms may include bacteria, yeast, and molds, all of which can contribute to food or beverage spoilage. Some microbial contaminants are also pathogenic and can cause illness when ingested, making it imperative to eliminate them from products intended for consumption.
Common heat-resistant microorganisms
Some of the most common microorganisms responsible for contamination within the food and beverage industry are well-known, due to outbreaks and products recalls, and include, among others:
- Salmonella, which causes the classic “food poisoning” and is linked to inadequately cleanliness and sterilization;
- Clostridium botulinum, which is often associated with canned goods that have been improperly preserved; and
- Coliform bacteria such as E. coli, which are typically introduced through fecal contamination.
An awareness of the types of contaminating microbes that are likely to be present in your product is important, as microorganisms have varying levels of susceptibility to heat-mediated killing. Generally speaking, gram positive bacteria are more resistant to heat than gram negative bacteria such as the three examples cited above, so if your product has a history of contamination with gram positive bacteria, a more intense pasteurization process may be needed. Similarly, bacteria that form spores are more resistant to heat than those that do not, and require higher temperatures and/or longer pasteurization time to adequately eliminate.
Another factor to consider is the nature of the product itself, as product composition can affect how easily or quickly microbial contaminants are killed. For example, the pH of a solution can have a marked effect on the speed of bacterial killing, with pasteurization being more effective at high and low pHs and less efficient at mid-range pHs. The presence of oil, fat, and other substances in the product can also affect the efficiency of pasteurization processes, highlighting the importance of designing a pasteurization protocol that is specific to and appropriate for your product.
How to determine the optimal pasteurization settings
The principle of the pasteurization procedure resides in reducing the bioburden by x logs by applying heat for a defined amount of time. A key parameter in designing a pasteurization process is determining the optimal duration of heat application that is needed to achieve the desired level of microbial killing.
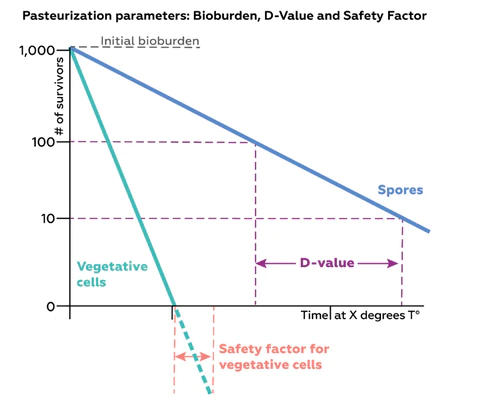
Generally speaking, this time period is defined as follows:
i) The time needed to reduce the bioburden of the most resistant organism in the solution by 1 log; or
ii) The time needed to reduce the maximum bioburden in the product by 1 log.
iii) Target number (N) of survivors after pasteurization (N = 1, when the target number of survivors is 0, as in the example above)
T = D value x log (B/N)
Once this calculation has been made, it is important to add a safety factor (SF), which is an additional period of treatment beyond what was calculated to be strictly necessary, as an extra buffer against leaving residual contamination. As a general rule of thumb, an SF of 6 log is considered sterilization.
When selecting a target SF, keep in mind that an intermediate value is probably best, as aiming for a high SF can result in undesirable degradation of the product; in the case of food and beverage products, applying too much heat or for too long can negatively affect their sensory properties, such as color, taste, and smell.
Aiming for an overly high SF can also reduce the efficiency of the process, as it requires more energy and time than a briefer pasteurization step. On the other hand, it is important not to underestimate the optimal SF, as applying inadequate heat or for too short a time can result in residual contamination of the product.
The best way to go about defining the appropriate SF for your product and ensuring that the pasteurization process is achieving its intended goal is through process monitoring.
Monitoring pasteurization efficacy
Monitoring process input and output is crucial for ensuring that the selected pasteurization conditions are performing adequately and that no unexpected contamination events take place throughout the process or over time. Regularly testing the production chain at different stages can provide a snapshot of the overall level of microbes in the product, as well as sound an early warning in case of unanticipated issues.
The first step is to monitor and record the bioburden of the original, unpasteurized, product: that is, to determine how many microbes are going into the process. To accomplish this, we recommend routinely testing products before they enter the process and maintaining a complete and detailed log of the prepasteurization data history.
After the pasteurization process is complete, the product should be monitored systematically to ensure that the target bioburden reduction has been achieved, and that the selected parameters are still appropriate and effective. As with the prepasteurization levels, it is important to maintain a clear record of these values as well, to have a comprehensive picture of the process performance over time. If the product will be subjected to other steps postpasteurziation, such as packaging, it is advisable to also monitor the packaging environment and equipment to check that the product does not become recontaminated after the pasteurization step.
Regular monitoring of the production environment, processes, and equipment is key to improving process efficiency and product conformity, as well as ensuring that your industrial processes are safe and produce products of consistent quality.
Conclusion
- The aim of pasteurization is to reduce the bioburden of a product through heat-mediated microbial killing. Designing an optimized pasteurization process will help not only maximize product appeal and avoid spoilage, but will also reduce resource wastage.
- When optimizing a pasteurization process, keep in mind that an overly high SF may unnecessarily deteriorate the product and/or degrade process efficiency, while an overly low SF may result in residual contamination of the product.
- It is important to regularly check the prepasteurization bioburden to verify that the pasteurization step will have the intended effect.
- It is crucial to monitor the cleanliness of the process environment and equipment postpasteurization to ensure the product does not become recontaminated between the pasteurization and filling steps.


